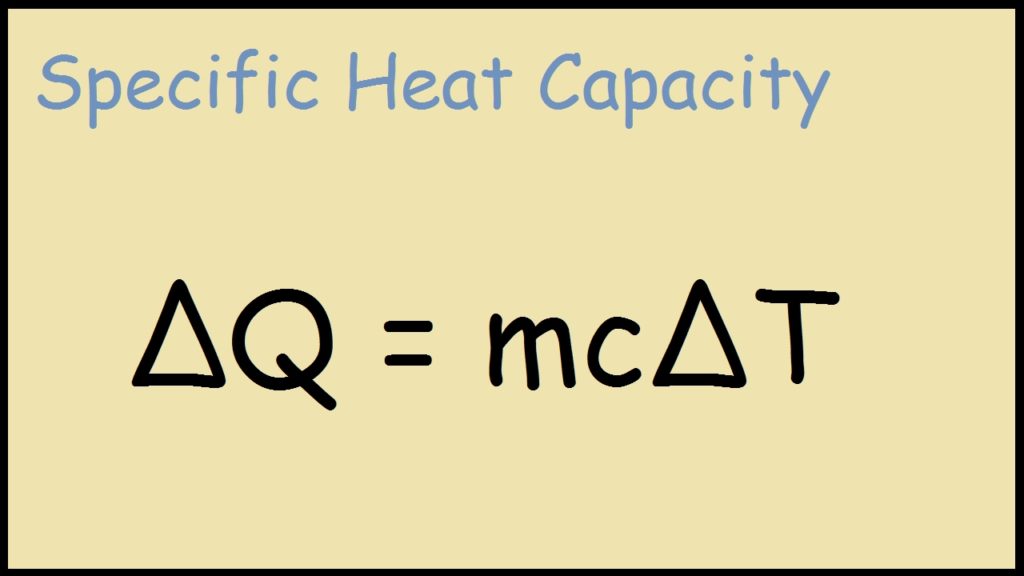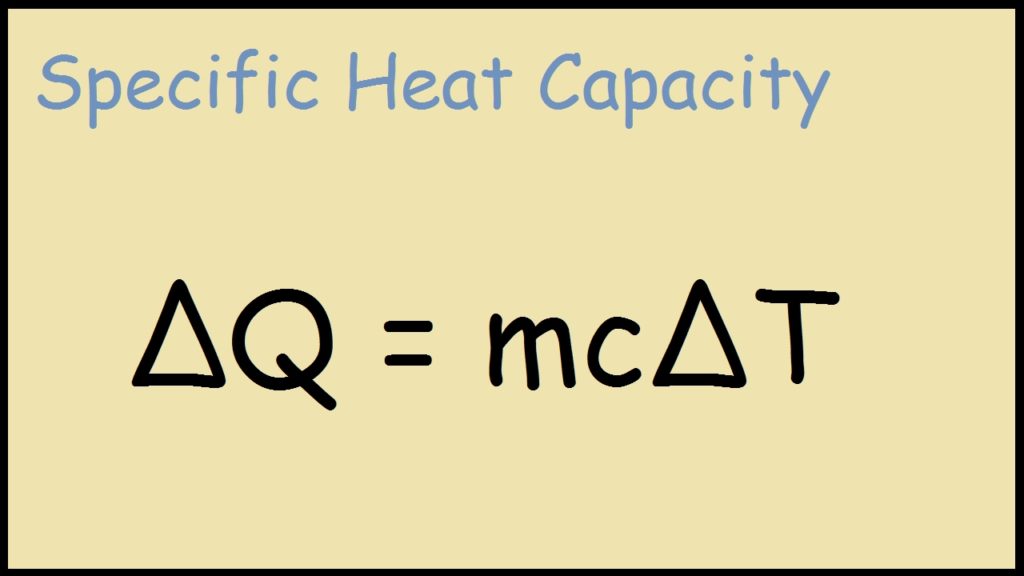Water has a specific heat capacity of 4 18 j or 1 calorie gram c.
Specific heat capacity process.
Anonymous user physics 28 oct 2019 1133 views.
We all have heard of specific heats of gases pertaining to constant volume and constant.
1 the change in temperature 2 the mass of the system and 3 the substance and phase of the substance.
Thank you for the answer request.
The specific heat capacity of a monoatomic gas for the process t v v constant is where r is gas constant.
In contrast copper has a specific heat capacity of 0 39 j.
Experiments show that the transferred heat depends on three factors.
The heat capacity may be negative zero positive or infinite depending on the process the system undergoes during the heat transfer.
The specific heat capacity of a monoatomic gas for the process t v v constant is where r is gas constant.
C q mδt q is the amount of supplied or subtracted heat in joules m is the mass of the sample and δt is the difference between the initial and final temperatures.
The si unit of heat capacity is joule per kelvin j k.
In the section 4 7 of the 7th edition of the book heat and thermodynamics written by m.
Specific heat capacity examples.
This is a much higher value than that of most other substances which makes water exceptionally good at regulating temperature.
This quantity is known as the specific heat capacity or simply the specific heat which is the heat capacity per unit mass of a material.
Heat capacity or thermal capacity is a physical property of matter defined as the amount of heat to be supplied to a given mass of a material to produce a unit change in its temperature.
Heat capacity is an extensive property the corresponding intensive property is the specific heat capacity dividing the heat capacity by the amount of.
Heat capacity has a definite value only for a definite process.
Short answer specific heat at 1.